Erythropoietin (EPO), albumin, interferon or coagulation factors VII and VIII are some examples of proteins used for therapeutic purposes. These biotherapies are a relatively recent class of drugs that has significantly modified the management of genetic diseases (hemophilia, rare diseases such as Gaucher disease, Pompe disease ...) but also cancers. The development of such molecules has been delayed because of the induced immune reaction (we speak of immunogenicity) with the possible consequence of reducing their effectiveness, or even of causing autoimmune reactions that are dangerous for the patient. To limit immunogenicity, the therapeutic protein production strategy has improved over time. Proteins extracted from animal tissues were replaced by recombinant proteins and then by "humanized" proteins. Strategy most often paid. However, humanized proteins may cause an immune reaction. This is the case of relaxin which has been evaluated for its anti-inflammatory properties in indications such as heart failure and scleroderma.
Relaxin, a two-chain peptide hormone structurally related to insulin, is secreted by the corpus luteum and placenta during pregnancy to help uterus to adjust to the embryo development. Multiple injections of relaxin, as applied in systemic sclerosis, led to a high immunization rate. After chronic administration, anti-relaxin antibodies were detected in almost half of scleroderma subjects. Researchers from SIMOPRO (CEA-Joliot) and SANOFI sought whether this production of anti-relaxin antibodies could originate from the presence of relaxin specific T cells in healthy subjects. A large relaxin-specific T cell repertoire was found circulating in blood of healthy subjects. The frequency of relaxin-specific T cells is in the range of immunogenic therapeutic antibodies such as infliximab, rituximab and adalimumab and above that of the nonimmunogenic therapeutic antibodies. In the absence of any external stimulation by relaxin injection, the presence of circulating relaxin-specific T cells appears to be the result of an escape from the thymic negative selection. As an endogenous hormone, relaxin circultates at very low concentration in the blood and probably at an insufficcient level to be detected in the thymus. Data suggest that relaxin is at least partially ignored by the immune cells during T cell ontogeny. relaxin-specific T cells are, therefore, poorly counterselected in the thymus and are potentially functional but remain inactive owing to insufficient signals of T cell activation. However, repeated injections of recombinant relaxin might provide sufficient local concentrations of the protein and inflammatory signals resulting from tissue damage provoked by the injection or provided by relaxin itself to initiate a T cell response and the subsequent anti-drug antibody response.
By quantifying and characterizing RLN2-specific CD4 T cells isolated from the blood of healthy donors, we provided an explanatory model to account for the clinical immunogenicity of RLN2. Our data also highlight the relevance of investigating the naive T cell repertoire to anticipate immunogenicity of therapeutic proteins in humans.
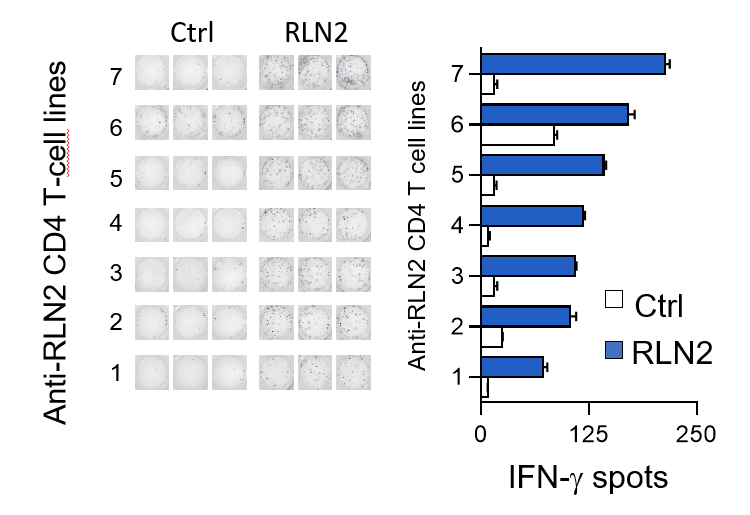
Détection des lymphocytes T CD4 spécifiques de la relaxine (RLN2) par amplification des cellules
in vitro et détection par Elispot IFN-g. © B. Maillère/CEA