Pluripotent stem cells are self-renewing, unspecialized cells capable of differentiating into any type of cell in the human body. In as much, they are a never-ending reservoir, but historically accessible only under certain conditions. Takahashi and Yamanaka recently demonstrated a methodology for reprogramming adult differentiated skin or blood cells back into the pluripotent state3. The resulting cells are called induced pluripotent stem cells (iPSCs).
Neurodegenerative diseases, including Alzheimer's disease, are currently considered incurable. Beyond the impact on patients and their loved ones, these diseases raise important medical, economic and societal issues. Today's treatments reduce certain symptoms only partially and the scientific community encounters great difficulty in its attempts to develop new, efficacious therapies. Contributing to that difficulty is the current inability to diagnose these diseases early in their course, despite the fact that the biological characteristics of them may develop in patients at least 20 years before symptoms appear.
With the goal of overcoming these difficulties, the researchers from SEPIA, CellTechs and LEMM teamed to develop a novel approach for modeling the pathophysiological characteristics of Alzheimer's disease. By differentiating iPSCs, they obtained small spheres of cells, measuring only a few millimeters, but harboring very interesting properties. Indeed, these tiny, three-dimensional structures, termed neuroectodermic organoids but nicknamed "mini-brains", reconstitute the different types of cells normally found in human brain tissue: neurons, astrocytes, glial cells, etc.
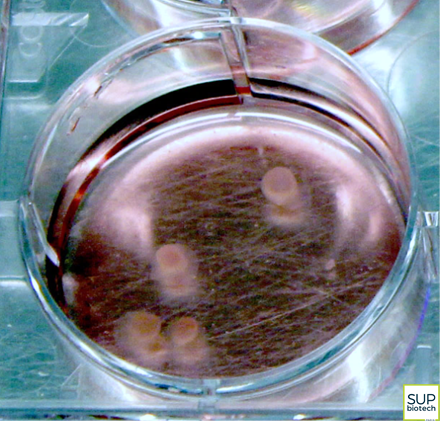 | 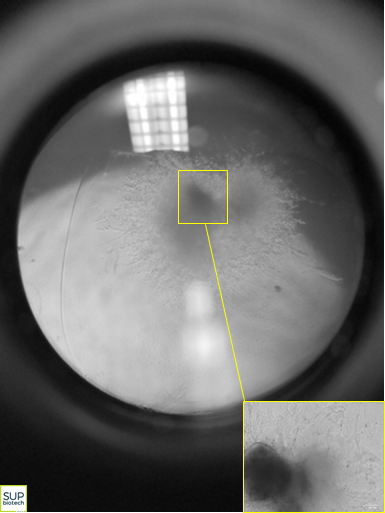 |
Then, within the organoids, the team used an amyloidogenesis activator, Aftin-55 (amyloid-β forty-two inducer), to successfully induce a significant and progressive production of a neurotoxic peptide, amyloid Aβ-42, the excessive synthesis of which has been shown to be a major component of Alzheimer's disease4.
This novel method using iPSCs to create pathological mini-brains and simulate aspects of the complexity of cerebral tissue opens new vistas for research in Alzheimer's disease. The in vitro modelling approach will advance the scientific and medical community's understanding of the disease, enable the identification of markers for early diagnosis and empower the development of new therapies. It will also likely constitute an alternative to animal models for certain steps in the study of the disease.
This technological miniaturization furthermore suggests a broad range of possibilities for the development and validation of tomorrow's innovative, targeted medicines and therapeutic strategies.
The team's work has received financial support from the Investissement d'Avenir program 3D Neurosecure and the European JPND program 3DMinibrain.