In plants, algae and cyanobacteria, photosynthesis takes place in membrane-bound structures, the thylakoids. In addition to photosynthesis proteins, other proteins are located in or in the immediate periphery of these membranes and can interact with the photosynthetic chain. Among them, the enzyme PTOX (plastide terminal oxidase), discovered in the 1990s, is of interest.
This enzyme catalyses the oxidation of plastoquinones, coenzymes that participate in the electron transport chain necessary for photosynthesis. PTOX is believed to have several essential functions and may compete with linear and cyclic electron flow. PTOX activity is strongly linked to its localization but the mechanisms regulating it are not known. In a previous study conducted in Arabidopsis thaliana, researchers from the Fundamental Mechanisms of Bioenergy Laboratory (LMB- I2BC) showed that PTOX was associated with thylakoid membranes isolated from leaves exposed to high light intensity but not in those isolated from dark-adapted leaves. The authors hypothesized that the attachment of PTOX to the membranes depends on the proton motive force, i.e. the electrochemical membrane potential difference generated by the active translocation of protons across the membrane by the photosynthetic electron transport chain.
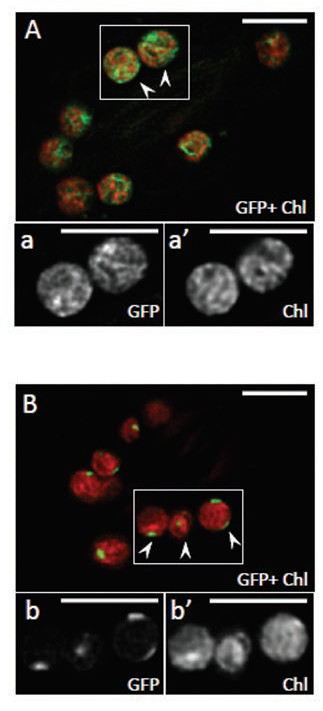
The LMB team joined forces with researchers from IMAPP (I2BC/ Institut Joliot), the Institut de Biologie Paris Seine and IRIG to confirm this hypothesis. They investigated the effect of uncouplers (ionophores) on the association of PTOX with thylakoid membrane, which, by allowing certain ions to pass through the membrane, reduce the proton motive force. They found that the presence of these ionophores in Arabidopsis leaves exposed to high light leads to partial dissociation of PTOX at the membranes. In vitro reconstitution experiments show that binding of a purified recombinant form of PTOX to liposomes or isolated thylakoid membranes is enhanced at slightly alkaline pH values or in the presence of millimolar concentrations of KCl or MgCl2. The researchers then showed that PTOX (recombinant GFP-bound protein) is fairly equally distributed in a network-like structure in leaves incubated in high light. In dark-adapted leaves, the distribution is very different: PTOX forms distinct spots in the chloroplasts, suggesting that the protein accumulates in places. Infiltration of the leaves with ionophores induces the same type of heterogeneous localization.
The authors propose a dynamic PTOX association with the thylakoid membrane depending on the presence of a proton motive force. In light, photosynthesis takes place and generates a proton motive force that will favour the strong association of PTOX with the membranes; in darkness, photosynthesis does not take place, the proton motive force is reduced and the PTOX association with the membranes is much weaker and it accumulates in certain spots.