Some drugs can destroy liver cells. This hepatotoxicity is difficult to predict. However, its assessment is of paramount importance in the development of a drug. Although the mechanisms are poorly understood, it is increasingly accepted that certain hepatobiliary transporters that allow the elimination of drugs by biliary excretion are involved in these toxicity phenomena. For example, it is known that a functional deficiency of the MRP2 (Multidrug resistance-associated protein 2) transporter, responsible for the secretion from the liver to the bile of a large number of xenobiotics and endogenous substances, can cause an abnormal accumulation of these substances in the liver and generate hepatotoxicity. Conventional pharmacokinetic approaches, which rely mainly on the dosage of drugs and metabolites in biological fluids, do not allow the hepatic transport capacity of MRP2 to be assessed in humans.
Pharmacologists from the BioMaps unit and the University of Vienna (Austria) have repositioned 99mTc-mebrofenine, a radiopharmaceutical widely used for hepatobiliary gamma-scanning, as a molecular imaging probe. Indeed, 99mTc-mebrofenine is a substrate for MRP2 and has the advantage of not being metabolized. PK models were developed to quantify MRP2 transport capacity under different conditions of pharmacological inhibition. In particular, the researchers were able to show that targeted inhibition of MRP2 can be achieved with certain drugs, including very low doses of cyclosporin, without modulating the activity of other transporters or hepatic perfusion as measured by ultrasound imaging.
The combination of clinical imaging modalities and selective pharmacological inhibition of MRP2 is achievable in humans in a hospital setting. This pharmacokinetic imaging strategy should make it possible to study the regulation of the functional reserve of MRP2 transport in different pathological situations in order to provide a mechanistic and molecular explanation for certain hepatotoxicity phenomena that are still poorly explained.
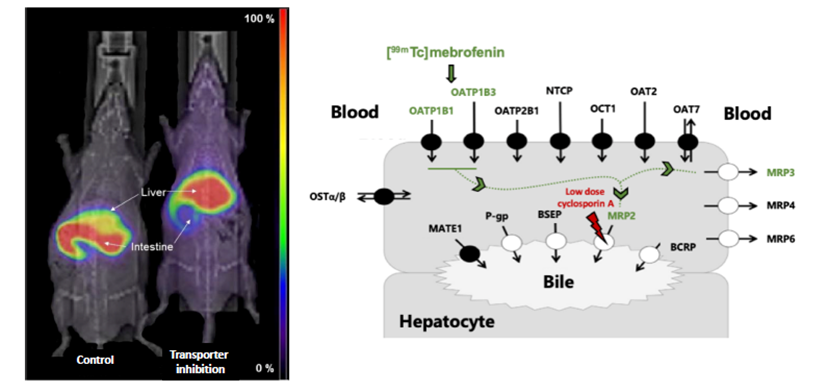
Scintigraphic imaging using 99mTc-mebrofenin probe makes it possible to study the activity of certain hepatic transporters through the development of targeted pharmacological inhibition protocols. © N. Tournier/CEA
Contact:
Nicolas TOURNIER (nicolas.tournier@cea.fr)