The pharmacological characterization of the interactions between the different peptides (animal toxins, hormones...) or small molecules (phycotoxins) studied in the laboratory and their respective molecular targets is performed using in vitro, ex-vivo and in-vivo experiments. These studies are crucial in the understanding of the mode of action of these molecules and to assess their potential development as innovative drugs according to their pharmacological profile.
1 - Binding experiments (GPCRs, nicotinic acetylcholine receptors (nAChRs), ion channels)
Our ability to label (125I, 3H, 14C) and use radioactive molecules allows us to perform binding assays to characterize the fundamental parameters of toxin/target interactions by kinetic (Kon, Koff), saturation (KD, Bmax) or competition experiments (IC50, Ki). In addition, binding assays allow to study the mode of action (competitive/non-competitive antagonism, allostery) and the determination of the pharmacological profile (subtype and species selectivity) of studied compounds (Blanchet et al., 2017; Petrel et al., 2013).
2 - Functionnal characterization
For the functional study of ligands interacting with GPCR and characterization of their mode of action (agonist/inverse agonist/antagonist/allosteric), several techniques are implemented in the laboratory, most of them based on fluorescence/luminescence measurement techniques. Two dedicated microplate readers are available (BMG Labtech Clariostar and Perkin Elmer Envision Xcite) allowing the measurement of fluorescence, FRET, time resolved-FRET, BRET or luminescence in 96 and 384 well plates.
The second messengers, cAMP and inisitol-phosphate (IP-1), as well as activation of downstream kinases such as ERK1/2 MAP kinases, are quantified by TR-FRET using commercial hTRF kits. In addition, modulation of intracellular Ca2+ concentration is monitored by the means of calcium-sensitive fluorescent probes (Fluo3, Fluo4 …), while recruitment of beta-arrestins by GPCRs is quantified by BRET technique, using YFP-tagged receptors and luciferase-tagged b-arrestins.
Finally, receptor oligomerization is studied either by TR-FRET using SnapTAG tagged receptors labelled with terbium cryptates and compatible fluorescence acceptors or by BRET (receptors tagged with RLuc8 and Venus) and NanoBiT technologies (receptors tagged with SmBit and LgBit fragments of the NanoLuciferase) (Ciolek et al., 2017; Reynaud et al., 2020).
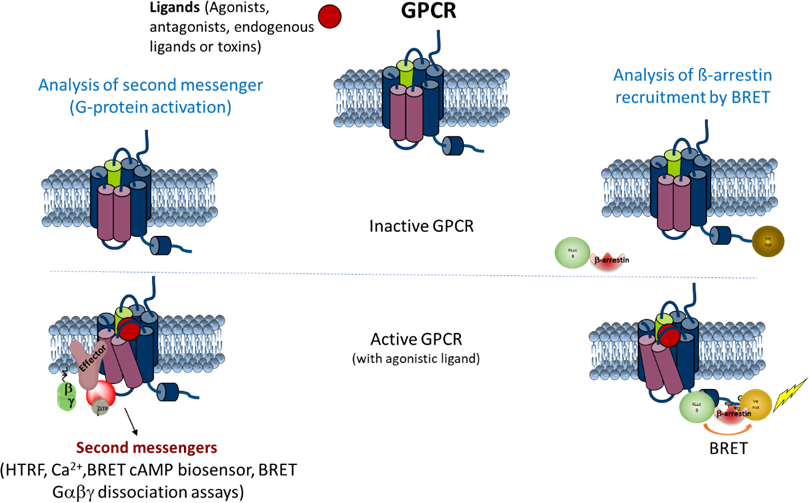
Functional characterization of the interactions between ligands and GPCRs