Melanoma is a rare skin cancer (2 to 3% of cancers). Its annual incidence has been steadily increasing for 50 years. Melanoma derives from melanocytes, the pigmented cells of the skin. Successive mutations in its genome give melanocyte the ability to multiply infinitely and transform it into malignant cell. The geneticists have found the oncogenic mutation V600E on the B-RAF gene in one melanoma out of two. But the mechanisms supporting the malignant transformation of melanocytes are complex and far from well understood. First observation: when expressed in cells without other cancer-associated mutations, the B-RAF-V600E mutation stops the proliferation in a particular and potentially definitive way. This is the so-called senescence. The moles are also composed of senescent melanocytes, mostly because of the expression of B-RAF-V600E. In the vast majority of cases, these moles remain benign which shows the efficiency of senescence to block tumor development. However, and this is the second observation, more than a quarter of melanomas develop from a mole. Thus, melanocytes carrying the B-RAF-V600E mutation should escape their senescence and start again or continue to proliferate to be able to transform into malignant cells. It is hypothesized that additional mutations (linked to UV exposure, for example) are required.
A
team from SBIGEM (I2BC@Saclay) is studying the mechanisms of cell senescence triggered by B-RAF-V600E or other stresses such as DNA damage. She has created model lines of human cells in which it is possible to modulate the production of mutant B-RAF-V600E. After a screening of a bank of molecules that showed glucocorticoid effect on senescence, they decided to study their effects on B-RAF-V600E-induced senescence. For this study, they collaborated with teams from SCBM (Joliot Institute) et du CNRGH (Jacob Institute).
In
an article published in the Journal of
Cell Science, researchers show that a glucocorticoid, clobetasol, delays or
bypasses the entry into senescence induced by the expression of the B-RAF-V600E
mutation in human fibroblasts but that this compound is unable to bring senescent
cells back to a proliferative state. Glucocorticoids are steroidal hormones
that bind to a receptor (the glucocorticoid receptor or GR) and relocate it to
the nucleus. The GR then acts on the expression of many genes (transcription),
positively or negatively. The researchers performed transcriptomic analysis and
siRNA knock-down experiments to identify potential target genes for clobetasol
action against senescence. They found the EGR1 gene, which encodes a
transcription factor, that is, a protein that is itself involved in the
regulation of transcription. EGR1 is persistently expressed because of the
V600E mutation of B-RAF. Among its targets, researchers have specifically
identified two genes involved in cell proliferation arrest. To summarize, the
authors have shown that EGR1 is important for rapid arrest of proliferation in
cells that express B-RAF-V600E, that it is a target of glucocorticoids, but
also that glucocorticoids target other genes that are yet to be identified to
explain all of their effects. (see figure).
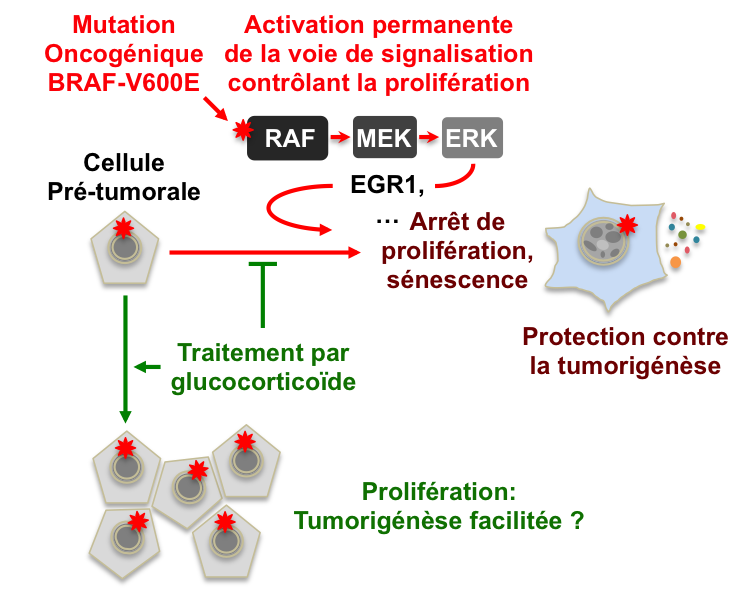
Next step is to characterize the effect of glucocorticoids and the role of EGR1 in human melanocytes that express B-RAF-V600E. This work also encourage a more precise evaluation of the effect of glucocorticoids on neoplasias and tumors known to be induced by the B-RAF-V600E mutation (melanomas, but also certain thyroid cancers and colorectal cancers).