The
intestinal microbiota, or intestinal flora, is considered
an organ in its own right. Not only does it play a role in the body's immune and metabolic functions, but in recent years it has become evident that it also
plays a role in brain function. Many studies are thus examining the
links between imbalances in the microbiota (dysbiosis) and psychological disorders. Of these, depression affects over 300 million people and is the leading cause of disability worldwide.
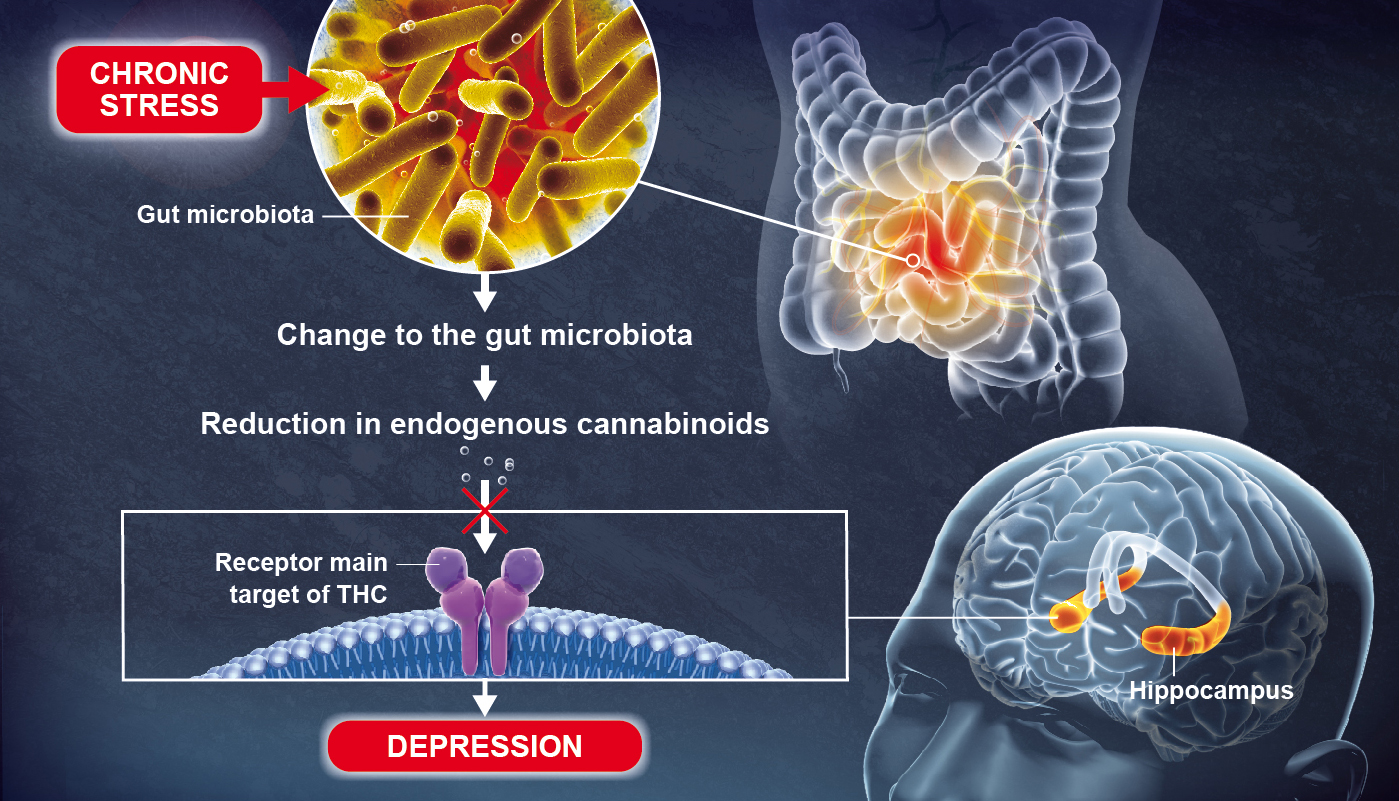
In this study, the researchers dissected the
link between depressive disorders and alterations in the gut microbiota in mice. To do this, they transplanted to naive mice the microbiota of healthy mice or mice with mood disorders induced by mild chronic stress. Then, in these mice, they
studiedthe microbiota and the metabolism of polyunsaturated fatty acids by SFC-MS[2] (contribution of DMTS-SPI)
as well as
neurogenesis in the hippocampus, a brain region strongly involved in the development of symptoms. depressive.
The results obtained show that
the symptoms of stressed mice (decrease in neurogenesis in the hippocampus and mood disorders)
can be transferred to naive recipient mice by transplantation of fecal microbiota. Metabolomic analysis reveals that
the recipient mice have an impaired fatty acid metabolism, characterized by
deficits in lipid precursors of endogenous cannabinoids[3], which lead to impaired activity of the endocannabinoid system in the brain (figure). The
undesirable effects of this microbiota transfer can be
attenuated by increasing the level of endogenous cannabinoids via the pharmacological blocking of the enzymes that degrade them or by supplementing the diet with a precursor of endogenous cannabinoids. Finally, the study shows that
chronic mild stress induces dysbiosis of the intestinal microbiota in mice characterized by a decrease in the abundance of lactobacilli, also observed in recipient mice. Supplementing the diet of stressed mice with a strain of lactobacillus increases both brain endogenous cannabinoid levels and hippocampal neurogenesis, thereby reducing mood disturbances.
© Institut Pasteur / Pascal Marseaud, with their kind permission
In conclusion, these observations strongly suggest that the link between microbiota dysbiosis and mood disorders lies in the endocannabinoid system. In addition, this study supports the concept that dietary or probiotic interventions could be effective levers in the therapeutic arsenal to fight against depressive syndromes associated with stress.
[1] The metabolome is constituted by all the metabolites of a given biological sample.
[2] SFC-MS, supercritical chromatography coupled with mass spectrometry
[3] Endogenous cannabinoids are fatty acid derivatives.