An object is said to be chiral if it cannot be superimposed on its image in a plane mirror, like for example a hand. A molecule can be chiral; it then exists in two forms, called enantiomers, which deviate the plane of polarization of polarized light in opposite directions. For compounds whose chirality is based only on the presence of different isotopes of hydrogen (hydrogen, deuterium or tritium), this optical property cannot be measured: these chiral compounds possess crypto-optically properties.
The smallest representative of these molecules is the radioactive chiral methyl (C1H2H3H), which proved to be very useful as a probe for deciphering the reaction mechanism of methyl transferases. These enzymes, belonging to the alkyl transferase family, catalyze the transfer of a methyl group from a donor compound to a target acceptor compound, a key reaction allowing the functional modulation of a large number of substrates, ranging from small metabolites to macromolecules.
Other probes could be useful for studying other alkyl transferases, since their mechanism varies from enzyme to enzyme. However, due to the presence of a tritium atom, the manipulation of these radioactive chiral probes remains difficult and delicate and limited to a few laboratories. In addition, characterization of their properties, including the measurement of enantiomeric excess (ie enantio-purity) constitutes a real challenge.
In a study published in Organic Letters, chemists of DMTS (SCBM) synthesized and characterized a deuterated, non-radioactive chiral probe containing a chiral ethyl group (C1H2HCH3), [2H] -ethyl tosylate (figure). This synthesis, a true chemical feat, led to the production of the two enantiomers with a maximum incorporation of deuterium atoms at the desired position. A second challenge consisted in characterizing their chiral properties. For this, the authors used two advanced spectroscopic tools. With deuterium NMR in anisotropic medium made, the enantiomeric excess of each compound was determined to be greater than 90%: using an anisotropic medium (chiral orientation agent), the two enantiomers hitherto indiscriminate by NMR, have sufficiently different orientations to allow the separation of their NMR signals and the determination of their respective enantiomeric excesses. The absolute configuration of each enantiomer was defined thanks to vibrational circular dichroism, an extremely sensitive technique based on the small difference in absorption, in the field of vibrational transitions, by a chiral molecule of the right circularly polarized light and left.
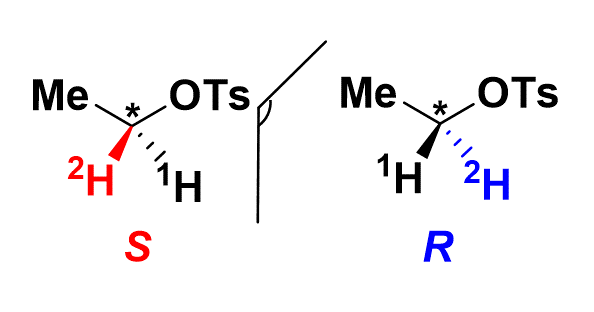
Figure : Structure of the two enantiomers of [2H] -ethyl tosylate,
synthesized and characterized in this study.
In conclusion, this study generated separately, via a high-performance synthesis, the two enantiomers of a non-radioactive chiral probe, with high enantiomeric excesses. Easier to manipulate than radioactive ones, this probe will be useful for studying enzymes such as alkyl transferases. Understanding their reaction mechanism is indeed essential for the development of new biocatalytic and therapeutic applications.