| |
2024
| |
|
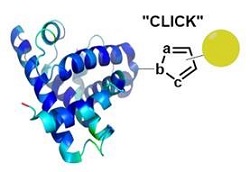
Click and bioorthogonal chemistries for health The development of chemical reactions that can be performed in living systems (i.e. cells, model organisms) has long held unique fascination in the field of chemical biology. A bio-orthogonal reaction is characterized by the reaction of two functionalities, which will react under mild physiological conditions and are inert towards the biological environment. On the other hand, the discovery of chemical reactions fulfilling the criteria of the click chemistry concept continue to have a huge impact in many research fields including heterocyclic chemistry. Quintessential example is the copper-catalyzed azide-alkyne cycloadditions (CuAAC). Our laboratory is involved in the discovery and use of such reactions. Recent work from our team identified several mesoionic compounds as new efficient dipoles for click reactions with terminal alkynes and for bioorthogonal reactions with cyclic alkynes. These reactions were used both for biological and synthetic applications.
|
2023
|
|

| Denis Servent (DMTS / SIMoS), le 5 décembre 2023 denis.servent@cea.fr
Structural and functional diversity of venom toxins interacting with GPCRs
Peptide toxins from venoms have undergone a long evolutionary process allowing host defense or prey capture and making them highly selective and potent for their target. This has resulted in the emergence of a large panel of toxins from a wide diversity of species, with varied structures and multiple associated biological functions. In this way, animal toxins constitute an inexhaustible reservoir of druggable molecules due to their interesting pharmacological properties. One of the most interesting classes of therapeutic targets is the G-protein-coupled receptors (GPCRs) that represent the largest family of membrane receptors in mammals with approximately 800 different members. They are involved in almost all biological functions and are the target of almost 30% of drugs currently on the market. Given the interest of GPCRs in the therapeutic field, the study of toxins that can interact with and modulate their activity with the purpose of drug development is of particular importance. The presentation will focus on toxins targeting GPCRs, including peptide-interacting receptors or aminergic receptors, with a particular focus on structural aspects and potential medical applications. The toxins exhibit a great diversity in size, from 10 to 80 amino acids long, in disulfide bridges, from none to five, and belong to a large panel of structural scaffolds, including inhibitory cystine knot (ICK), three-finger fold, and Kunitz-type toxins. These toxins belong to two distinct families, (i) agonist-mimicking toxins that act as endogenous agonists targeting the corresponding receptor, and (ii) toxins that differ structurally from natural agonists and which display agonist, antagonist, or allosteric properties.
|
| Charles Truillet (SHFJ / BioMaps), le 6 juin 2023 charles.truillet@cea.fr
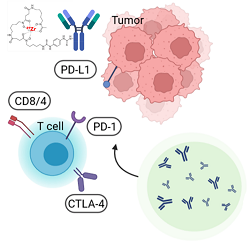
Antibody-based radiopharmaceuticals. Over the past 25 years, therapeutic monoclonal antibodies (mAbs) have emerged as the dominant treatment modality for a wide array of diseases. This remarkable rise in their utilization can be attributed to noteworthy technological advancements that have greatly accelerated and improved the process of discovering and developing mAb therapies, marking the advent of a new era characterized by heightened efficiency and effectiveness.
However, while there have been promising results, the number of patients who truly benefit from the treatment remains limited. Moreover, significant challenges such as toxicity and treatment resistance continue to persist. To address these hurdles, we propose that immunoPET, a groundbreaking approach that combines the remarkable targeting specificity of monoclonal antibodies (mAbs) with the exceptional sensitivity and resolution of Positron Emission Tomography (PET) imaging, could serve as a breakthrough. ImmunoPET has the potential to revolutionize the assessment of potent biomarkers related to drug-based antibody distribution and pharmacokinetics, as well as enable the development of specific radioligands for evaluating protein markers or immune cell populations. To address these questions, it is crucial to employ pharmacokinetic modeling approaches and implement a meticulous design process that encompasses the entire journey from the antibody scaffold to the radiolabeling process. These essential steps are paramount in tackling these challenges and finding effective solutions.
|
| | Stéphanie Simon (DMTS / SPI), le 9 mai 2023 stephanie.simon@cea.fr
Monoclonal antibodies in the field of in vitro diagnostics
Monoclonal antibodies (mAbs) have significant interest and utility in the field of in vitro diagnostics (IVD). They are highly specific for their target antigens and can be easily available from cloning a single B-cell that will produce a unique antibody, ensuring that each monoclonal antibody recognizes a specific epitope on the target molecule. This specificity allows for accurate identification and quantification of specific analytes in a sample, minimizing false-positive or false-negative results. mAbs can be designed and engineered to have high sensitivity, enabling the detection of low concentrations of analytes in a sample. This is particularly important in diagnostic tests where detecting small amounts of target molecules, such as biomarkers or infectious agents, is crucial for accurate diagnosis and monitoring of diseases. mAbs also offer a standardized and reproducible reagent for diagnostic assays. Since they are produced through controlled manufacturing processes, their quality and performance characteristics can be precisely defined and maintained. This ensures consistency and reliability of test results across different laboratories and over time. Thus, mAbs can be utilized in multiplex assays, where multiple analytes can be simultaneously detected in a single sample. By using different mAbs labeled with distinct markers, it becomes possible to measure various targets within the same assay. This multiplexing capability improves efficiency, conserves sample volume, and reduces time and costs associated with running multiple individual tests. Additionally, mAbs have a long shelf life, making them suitable for commercial diagnostic kits. They can be stored for extended periods without significant loss of activity or specificity, ensuring the availability of reliable diagnostic reagents when needed. Overall, mAbs provide highly specific, sensitive, and standardized reagents for in vitro diagnostic tests, enabling accurate and reliable detection of target analytes. Their versatility and compatibility with various assay formats make them indispensable tools in the field of diagnostics, aiding in disease diagnosis, prognosis, treatment monitoring, and research applications.
|
| Bernard Maillère (DMTS / SIMoS), le 4 avril 2023 bernard.maillere@cea.fr
The varied landscape of the therapeutic antibodies
Antibodies are proteins naturally produced by the immune system to protect us from infection by pathogens. Their main characteristic is their affinity and selectivity for their target, which is harnessed to produce new drugs (8 of the top-10 highest-selling drugs are today antibodies). Full-length Antibodies are heterodimers of one heavy chains (450 aa) and one light chains (212 aa). In the body, antibodies are produced by B-lymphocytes and their antigen specificity is the result of three mechanisms, namely i) generation of a wide repertoire of antibodies resulting from somatic gene recombination and mutations ii) selection of clones by the contact with the antigen and expansion of the specific clones. These mechanisms are advantageously used to produce antibodies with a defined specificity either immunized animals or patients (Cell fusion, human B cell cloning) or from antibody libraries screened in vitro (Phage display, Yeast surface display). While selectivity of the antibodies relies on the variable domains, the constant domains confer to the full-length antibodies a very long half-life of 15 days in the body and can provide cytotoxic activities to immune cells.
Trademarked antibodies exhibit antagonist, agonist or cell deleting activities. Multiple formats exist with reduced size such Fab and nanobodies. Antibodies are very flexible molecules as they can be associated to a toxic moiety (the term ADC for Antibody-Drug-Conjugate is used) and can combine two specificities to be bi-specific. Selected examples are : i) the anti-CD19/CD3 antibody Blinatumomab (BlincytoTM, Amgen) used today as monotherapy for the treatment of adults with CD19 positive relapsed or refractory B-precursor acute lymphoblastic leukaemia (ALL); ii) Emicizumab (HemlibraTM, Roche), a bispecific factor IXa- and factor X-directed antibody, used to prevent or reduce bleeding in patients with haemophilia A (an inherited bleeding disorder caused by lack of factor VIII).
|
2022
| |
| Marie-Pierre Heck (DMTS / SCBM), le 6 décembre 2022 marie-pierre.heck@cea.fr
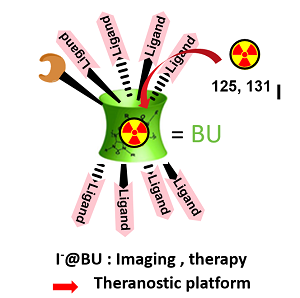
Conception and study of macrocyclic structures as new multivalent platforms for biological applications. Anions and cations are ubiquitous in nature and play very important roles in many areas, such as biological research, clinical diagnosis, industries and environmental process. There recognition and monitoring are of primordial importance in biological mechanisms, medicine and environment. The design of anion receptors is particularly challenging due to their larger size than isoelectronic cation, their strong hydration, their variety of geometries, and their sensitivity to pH. Despite numerous progress have been achieved, selective anion recognition is a challenge, especially in water and biological media due to the intrinsic characteristics of anions. Our research group has designed powerful receptors of anions based on bambusuril skeleton and evaluated their affinity towards halides. These neutral cavitands with a jigger-like conformation are prepared from cheap reagents using an easy and fast synthetic procedure. We have shown that such molecules exhibit highly specific recognition for iodides in organic and aqueous media making them the most efficient complexing agents currently known for which applications as sensors and imaging agents can be envisaged. Moreover, we have shown that bambusurils can be used as multivalent plateforms to link glycosidases inhibitors derived from 1-deoxynojirimycin (DNJ). These neoglycobambusurils caging-anions have inhibitory constants in the nanomolar range.
|
| | Éric Doris (DMTS / SCBM), le 10 mai 2022 eric.doris@cea.fr
Nanometric Micelles for Drug Delivery & Catalysis
Medicine has been a field where nanotechnologies have shown great promise for diagnosis and drug delivery applications. The challenge of nanomedicine consists in carrying active molecules through the different biological barriers and reaching specific targets in an efficient and non-toxic way. With the advent of nanotechnologies, different carrier systems are now available. However, the development of small biocompatible carriers with high loading capacity, extended circulation time, and favorable biodistribution has several unanswered issues. This talk will give an overview of our findings regarding polymerized micelles obtained from original diacetylene-containing amphiphiles. Their chemical synthesis, assembly and characterization will be presented as well as some biomedical applications such as tumor imaging and drug delivery. Micelles were also valorized by our group for synthetic applications such as the promotion of the Huisgen 1,3-dipolar cycloaddition ("click" reaction) between alkynes derivatives and azido compounds in water. Some examples illustrating the potential of the micelles in the catalysis of the "click" reaction will be presented.
|
| | Bertrand Kuhnast (SHFJ / BioMaps), le 19 avril 2022 bertrand.kuhnast@cea.fr
Translational development of radiopharmaceuticals at Service Hospitalier Frédéric Joliot (SHFJ). The SHFJ (Orsay, France) is a translational and multimodal molecular imaging facility. It gathers on the same site two cyclotrons for the daily production of positron emitters, R&D and GMP hot labs for the manufacturing of radiotracers and radiopharmaceuticals, an experimental and preclinical imaging platform comprising PET, PET/CT, SPECT/CT scanners and ultrasound devices and finally PET/CT, PET/MRI and MRI clinical scanners. Beside the clinical nuclear medicine practice, intensive research programs are dedicated to the translational development of new radiopharmaceuticals and the support and "derisking" in drug development. During this seminar, after a rapid technical overview of our facility, I will illustrate these translational developments with two selected examples: i) [18F]DPA-714, a radiopharmaceutical to image neuroinflammation: from the identification of the chemical scaffold to the clinical trials; step by step development of a radiopharmaceutical. ii) Isotopic radiolabelling of dolutegravir, an antiretroviral drug, with fluorine-18 to decipher treatment limitations: hope and pitfall in the development of a complex radiosynthesis scheme and first images.
|