DNA mutations have
important consequences: from the genetic diversity necessary for
the evolution of species to diseases such as
cancer. They result from exposure to mutagens (ionizing radiations, UV, "free radicals", etc.) and/or from internal dysfunctions that affect DNA repair pathways.
How can we study these phenomena and their consequences or know precisely the mutagenic effect of a molecule when most mutation events remain largely hidden from the eyes of biologists due to natural selection?
To achieve this,
Julie Soutourina's team (Genome transcriptional regulation / Genome Biology Department /
I2BC / CEA-Joliot)
studies the consequences of the accumulation of mutations in a model organism, the yeast
Saccharomyces cerevisiae. The experiment consists of
multiplying a population of cells from a single ancestor under given conditions (e.g. in the presence of a molecule or when the genome of the ancestor contains a mutation of interest)
and isolating a single daughter cell after several generations (bottlenecks)
which itself becomes the single ancestor of a population of cells multiplied over several generations, etc.
Coupled with high-throughput sequencing of the genome, this type of experiment
makes it possible to map spontaneous mutations precisely and measure their rate of appearance, while characterizing their effects on the phenotype.
microfluidics coming to the AID
The problem is that mutation rates are generally so low that a large number of lines grown in parallel, with repeated human intervention over a long period of time, are required (typically 800 Petri dishes with human intervention every 2 days for more than 6 months need to be handled). How to make the experiment less tedious? The groups of Florent Malloggi (CEA-Iramis, NIMBE/Lions) and Julie Soutourina have joined forces to develop and validate an innovative microfluidic-based system that automatizes cell culture for these experiments.
In practice, F. Malloggi's team built a model microfluidic chip by photolithography comprising several types of microstructures: "micro-culture chambers", to allow yeast to multiply over several generations, mounted in series alternately with micro-channels constituting population bottlenecks that allow only one cell to pass through. When a population of cells has occupied the entire space of the first chamber, a single daughter cell can pass into the downstream chamber; this single daughter cell multiplies until its progeny occupy the entire second chamber; a single daughter cell then passes into the next chamber and so on. In addition to these two types of microstructures, there are also entry and exit points and additional channels for cell inoculation and fluids circulation (especially the culture medium). In total, on a microfluidic chip made of polydimethylsiloxane (PDMS, a polymer), the researchers etched 8 arrays in parallel so that 8 mutation accumulation lines could be grown simultaneously for 1 to 3 months with limited human intervention. The system has been validated by comparing two strains: one control and one deficient in base excision repair.
This unique tool, combined with high-throughput sequencing, opens the way for massive mutation accumulation experiments to study the mutational processes that cause disease and to identify mutagenic compounds relevant for medical and environmental research.
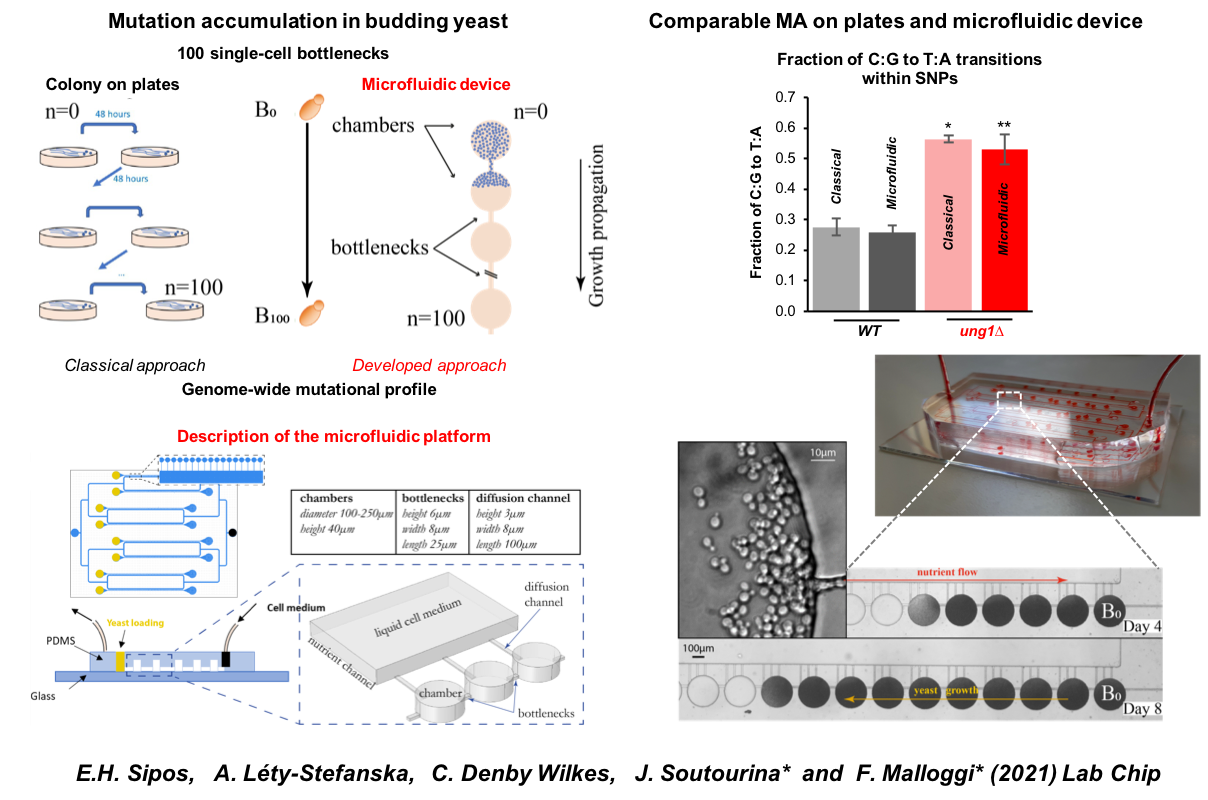
Device for mutation accumulation:
Top left - comparison of the usual manual petri dish technique and the microfluidic device; Top right: comparison of the 2 types of experiments; Bottom - description of the microfluidic device.
The work was supported by a programme of the CEA's Basic Research Division (DRF-Impulsion). The high-throughput sequencing was performed on the I2BC sequencing platform.
[1]
S. cerevisiae is a very good model: its genome, which is very close to that of humans (most cellular functions are conserved), is compact and the time between two generations is very short (about 90 minutes).