DESIGN OF A POWERFUL INHIBITOR OF HB-EGF FOR THE TREATMENT OF RAPIDLY PROGRESSIVE GLOMERULONEPHRITIS
Rapidly progressive glomerulonephritis is a rare kidney disease in which the filtration units of the kidneys, the glomeruli, are irretrievably destroyed. The disease is due to an (auto)-immune aggression of the glomeruli involving autoantibodies or antibacterial antibodies. The immune aggression activates the expression of a growth factor, HB-EGF, by podocytes, the specialized cells forming the filtration barrier of the kidney. As a result, the podocytes and glomerular parietal cells dedifferentiate and proliferate, destroying the glomeruli. Blocking HB-EGF protect the glomeruli despite the persistence of autoantibodies. Current treatments are based solely on immunosuppression and do no protect the kidney from HB-EGF. As a consequence, the disease is poorly managed leading to a high requency of terminal kidney failure or death.
The membrane precursor form of HB-EGF is also the natural receptor for the diphtheria toxin. Thus, we engineered, from a fragment of the diphtheria toxin, a powerful inhibitor of HB-EGF called DTR8, capable of blocking the proliferation of podocytes.
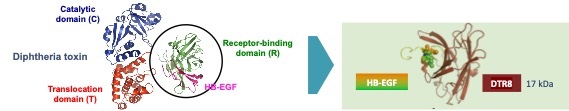
Left : structure of the diphtheria toxin bound to HB-EGF.
Right : DTR8 (brown) is an engineered receptor-binding domain of the diphtheria toxin carrying 12 mutations that improve its solubility and affinity for HB-EGF, and decrease its immunogenicity and antigenicity.
An initial proof of concept study in an animal model of the disease showed that DTR8 can protect the glomeruli and prevent kidney failure. Our goal is now to turn DTR8 into a drug for the treatment of rapidly progressive glomerulonephritis.
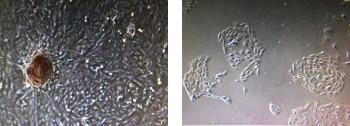
Left : podocytes from a pig kidney produce HB-EGF and proliferate as a result when put on a tissue culture plate.
Right : in the presence of DTR8, their proliferation is prevented.